Oxalyl Chloride
Oxalyl Chloride
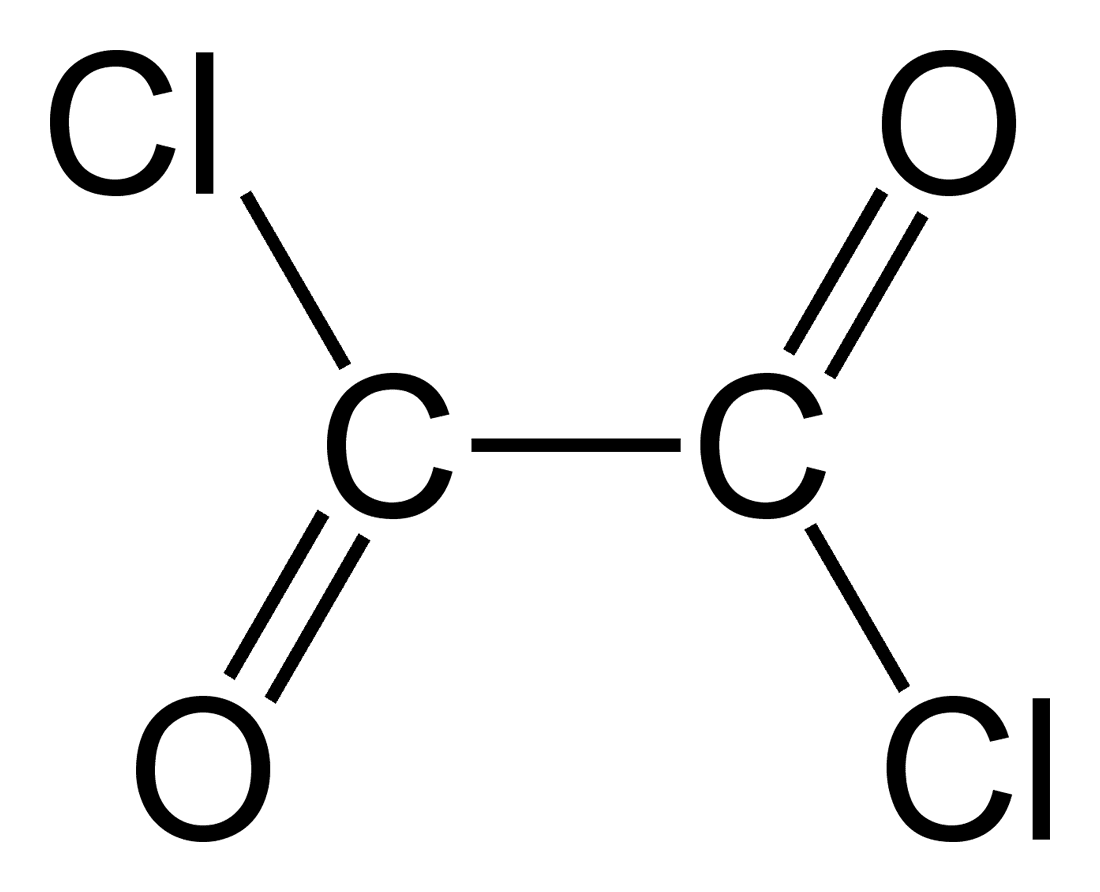
We are the manufacturer of oxalyl chloride (C2Cl2O2), a colorless liquid with a sharp odor that plays a vital role in organic chemistry. It functions as a versatile reagent, enabling various transformations in the synthesis of complex molecules.
In the pharmaceutical industry, oxalyl chloride serves as a key intermediate in the synthesis of numerous drugs and pharmaceutical ingredients.
Oxalyl chloride is utilized in the synthesis of agrochemicals such as herbicides, insecticides, and fungicides. it is employed in the production of certain polymers and materials. It participates in reactions leading to the formation of dye intermediates
Oxalyl chloride (C₂O₂Cl₂) is a versatile reagent used in organic chemistry for a variety of applications. Here are some of the main uses:
Conversion of Carboxylic Acids to Acyl Chlorides:
- Oxalyl chloride is commonly used to convert carboxylic acids to acyl chlorides.
- The reaction is beneficial because acyl chlorides are more reactive intermediates in subsequent reactions such as nucleophilic acyl substitution.
Formation of Esters and Amides:
- Through the formation of acyl chlorides, oxalyl chloride can facilitate the synthesis of esters and amides. For example, the acyl chloride formed can be reacted with an alcohol to form an ester or with an amine to form an amide.
Swern Oxidation:
- In the Swern oxidation, oxalyl chloride is used in combination with dimethyl sulfoxide (DMSO) and a base (usually triethylamine) to oxidize primary and secondary alcohols to aldehydes and ketones, respectively.
Vilsmeier-Haack Reaction:
- Oxalyl chloride can be used to generate a Vilsmeier reagent when reacted with DMF (dimethylformamide).
- The generated Vilsmeier reagent then reacts with the aromatic compound to introduce a formyl group.
Preparation of Acid Anhydrides:
- Oxalyl chloride can react with carboxylic acids to form acid anhydrides.
Generation of Chlorinating Agents:
- Oxalyl chloride is used in the generation of other chlorinating agents, such as phosphorus oxychloride (POCl₃), which can be used for chlorination reactions in organic synthesis.
Chemical Properties
- Molecular Formula: C₂O₂Cl₂
- Molecular Weight: 126.93 g/mol
- Appearance: Colorless to pale yellow liquid
- Boiling Point: 63-64°C (at 760 mmHg)
- Density: 1.48 g/cm³
- Solubility: Reacts with water, decomposing into hydrochloric acid (HCl), carbon monoxide (CO), and carbon dioxide (CO₂).
Handling and Safety
Oxalyl chloride is highly reactive and poses significant hazards:
- Toxicity: It releases toxic gases (HCl, CO, CO₂) upon decomposition. Inhalation of these gases can cause severe respiratory irritation and other health issues.
- Corrosivity: It is highly corrosive to skin and mucous membranes. Direct contact can cause severe burns.
- Reactivity: It reacts violently with water, alcohols, and other nucleophiles. Therefore, it should be handled under anhydrous conditions and in a well-ventilated fume hood.
Precautions
- Personal Protective Equipment (PPE): Appropriate PPE includes gloves, safety goggles, lab coat, and face shield.
- Storage: Oxalyl chloride should be stored in tightly sealed containers, away from moisture and incompatible substances.
- Emergency Measures: In case of exposure, affected areas should be flushed with plenty of water, and medical attention should be sought immediately.
In conclusion, oxalyl chloride is a powerful and useful reagent in organic chemistry with a range of applications, from synthesizing reactive intermediates to facilitating key oxidation reactions. Its handling requires strict safety measures due to its reactive and hazardous nature.
Anshul Specialty Molecules Private Limited
Flexcel Park, C Wing, 2nd Floor,S. V. Road, Near 24 Karat Multiplex, Jogeshwari (West), Mumbai 400 102. Ph : + 91 22 62252900.
